Set of Quantum Numbers
Quantum numbers are also used to understand other characteristics of atoms such as ionization energy and the atomic radius. Quantum number may be defined as a set of four numbers with the help of which we can get complete information about all the electrons in an atom ie location energy the.

Which Of The Following Sets Of Quantum Numbers Is Not Allowed Youtube
The principal quantum number n cannot be zero.

. Since each set is unique they serve as a way of uniquely naming individual electrons ie. Quantum Numbers Chemistry Questions with Solutions Q1. Value is a guess based on periodic table trend.
The first quantum number is called the principal quantum number. The story behind how these. Quantum numbersThese four quantum numbers are used to describe the probable location of an electron in an atom.
In atoms there are a total of four quantum numbers. Represented by n the principal quantum number largely determines the energy of an electron. The principal quantum number n the angular momentum quantum number l the magnetic quantum number ml.
0 1 2 3 and so on. The Principal Quantum Number The first quantum number describes the. There are four quantum numbers.
Write one set of quantum numbers n l ml ms that describe an electron in a 5p orbital. Principal quantum number n 2 Azimuthal quantum number l 1 Magnetic quantum number m -1 or 0 or 1. The three quantum numbers n l and m that describe an orbital are integers.
Electrons in the same atom that. When atoms gain lose or share. Notes on the Quantum Numbers of particular elements.
Which of the following sets of quantum numbers are correct. Four quantum numbers can be used to fully describe all of the properties of a given electron in an atom. In atoms there are a total of 4 quantum numbers.
The correct set of quantum numbers among the given options is. At this introductory level the equations are not needed. The correct set of quantum numbers for the unpaired electron of F atom is2 0 0 123 1 1 122 1 1 122 0 0 12The electronic.
Each one is a particular factor in an equation describing a property of the electron. The 4 quantum numbers. N ℓ m ℓ and m s.
A set of the four quantum numbers describes the unique properties of one specific electron in an atom. The allowed values of n are therefore 1 2. As you know four quantum numbers are used to describe the location and spin of an electron in an atom.
Quantum numbers are a set of 4 imaginary numbers which explain the position and spin of electrons in an atom it can not explain an atom as a whole Iodine has 53 electrons so there are. Principal quantum number n Azimuthal quantum number ℓ Magnetic quantum number mℓ Spin quantum number ms. This question has multiple correct options A n111m 12 B n211m 11 C n312m 12 D n314m 12 Medium.
Orbital angular momentum quantum. Here for this 2p electron the value of m depends on which orbital px or py. Principal azimuthal magnetic and spin quantum numbers are the 4 types of quantum numbers.
Their symbols are n ℓ m ℓ and m s. Four quantum numbers can describe an electron in an atom completely. Write all the possible sets of magnetic quantum numbers m_l for an.
So lets start with the first one the principal quantum number n which. EVERY electron in an atom has a specific unique set of these four quantum numbers. There are four quantum numbers.
Each electron in an atom has its own set of quantum numbers and no two electrons may have the same four quantum numbers according to the Pauli Exclusion Principle.

Which Set Of Quantum Number Is Not Possible For Electron Un 3 Rd Shell Youtube

Example Determining If A Set Of 4 Quantum Numbers Is Allowed Or Not Youtube

Quantum Number Orbital Definition Formula Diagram Shape

Quantum Numbers Valid And Invalid Sets Youtube
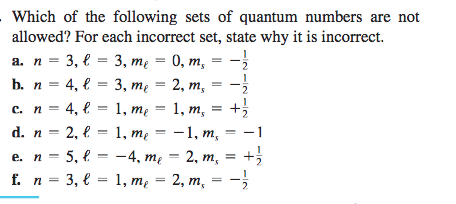
Solved Which Of The Following Sets Of Quantum Numbers Are Chegg Com

Solved From The Following Sets Of Four Quantum Numbers Chegg Com
Comments
Post a Comment